| Sign In | Join Free | My frbiz.com |
|
- Home
- Products
- About Us
- Quality Control
- Contact Us
- Get Quotations
| Sign In | Join Free | My frbiz.com |
|
Brand Name : Mcreat/OEM
Model Number : MC-151065
Certification : ISO13485/ FDA
Place of Origin : China
MOQ : 100pcs
Price : US$ 2.29-3/Piece
Payment Terms : T/T, L/C, D/A
Supply Ability : 50, 000PCS per Month
Delivery Time : 35-45 Days
Packaging Details : 10pcs/box 10boxes/carton
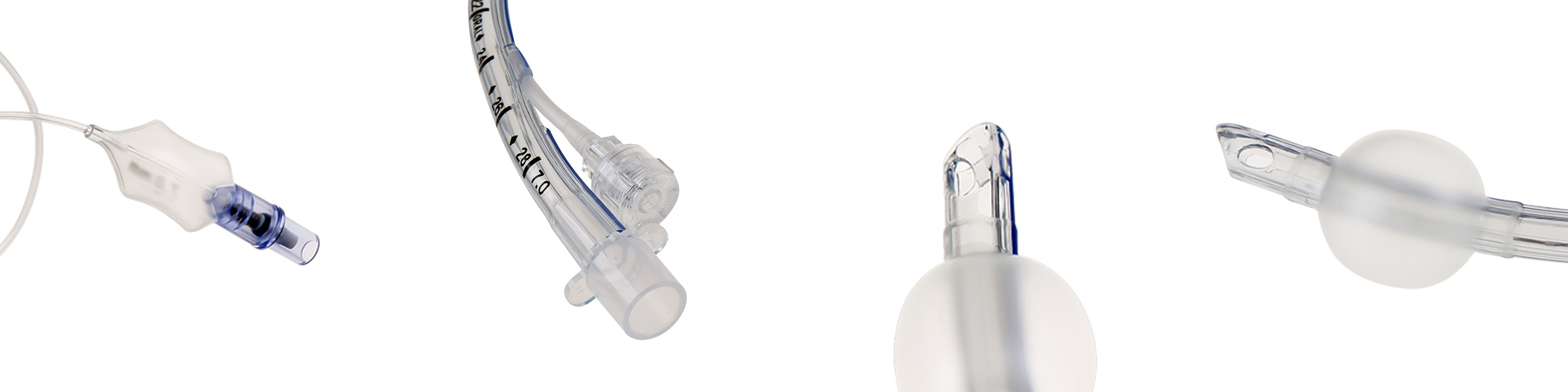
Name : Reinforced Visual Endotracheal Tube
Cuff Type : High Volume Low Pressure
Style : Regular or Reinforced
Specification : #6.0~#10.0
Color : Transparent
Usage : Single-use
steel : with
visual : yes
camera : with
Camera Use Kink Resistance Reinforced Endotracheal Tube Vision Tube
Feature
Endotracheal Tube is used to provide a direct and unimpeded airway for gases to pass to and from the lungs.
Kink resistant inflation tube to ensure patient safety during cuff inflation and deflation
Radio opaque line and markings provided to facilitate identification of tube position
Kink resistant, thin-walled, thermo-sensitive tube, softens at body temperature
Smooth inside/outside surface for ease in intubation, intubation and suctioning
Smooth polished murphy eye, distal end and tapered balloon end for atraumatic intubation and intubation
Camera Use Kink Resistance Reinforced Endotracheal Tube Vision Tube
Product Data Sheet
| CODE | ID(mm) | OD(mm) | Length(mm) |
| MC-151060 | 6.0 | 8.2 | 285 |
| MC-151065 | 6.5 | 8.8 | 295 |
| MC-151070 | 7.0 | 9.6 | 305 |
| MC-151075 | 7.5 | 10.2 | 315 |
| MC-151080 | 8.0 | 10.9 | 325 |
| MC-151085 | 8.5 | 11.5 | 325 |
| MC-151090 | 9.0 | 12.1 | 325 |
| MC-151095 | 9.5 | 12.7 | 325 |
| MC-151010 | 10.0 | 13.6 | 325 |
Abstract
The prevalence of obesity (body mass index (BMI) > 30 kg/m(2)) is increasing in both developed and developing countries, leading to a rise in the numbers of obese patients requiring general anaesthesia. Obese patients are at increased risk of anaesthetic complications, and tracheal intubation can be more difficult. Flexible intubation scopes (FISs) are recommended as an alternative method of intubation in these patients. Intubation with an FIS is considered an advanced method, requiring training and experience; therefore it may be underused in clinical practice. Patient outcomes following intubation with these scopes compared with other devices have not been systematically reviewed. We wished to compare the safety and effectiveness of a flexible intubation scope (FIS) used for tracheal intubation in obese patients (BMI > 30 kg/m(2)) with other methods of intubation, including conventional direct laryngoscopy, non-standard laryngoscopy and the use of intubating supraglottic airway devices. We aimed to compare the frequency of complications, as well as process indicators, such as time taken for intubation and the proportion of first attempts that were successful, between groups using the different methods of intubation. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and two trial registers on 18 January 2013, and performed reference checking and citation searching and contacted study authors to identify additional studies. We included randomized controlled trials (RCTs) of participants aged 16 years and older with a BMI > 30 kg/m(2) that had compared the use of an FIS for tracheal intubation with any one of three comparison groups: direct laryngoscopy; non-standard laryngoscopy (including indirect laryngoscopy using a videolaryngoscope (VLS) or a rigid or semi-rigid stylet); or intubation of supraglottic airway devices (SADs). We used standard methodological approaches expected by The Cochrane Collaboration, including independent review of titles, data extraction and risk of bias assessment by two investigators. Three eligible studies were identified, all comparing the use of an FIS with a VLS. All studies were small, with only 131 participants in total across all trials. It was impossible for the intubators to be unaware of the device used, so all studies were at high risk of performance and detection bias for outcomes related to intubation. Because of substantial differences in design between the studies, we did not combine their results in meta-analyses. The results for all outcomes were inconclusive, with no differences noted between FIS and VLS. Two studies with experienced intubators reported first attempt success rates greater than 70% in both groups and less than 5% of participants requiring a change of intubation device. No evidence was found of any difference in difficulty or time taken between FIS and VLS intubation. No serious complications or airway trauma was reported, so we were unable to address these outcomes. Bleeding was uncommon, occurring in less than 5% of participants, and we found no evidence that it was more likely in the FIS group. One small study with a novice intubator reported no successful intubations using an FIS and compared with the use of an intubating SAD and stylet, as well as with a VLS. With only five participants in each group, no conclusions can be drawn from these additional comparisons. The evidence base is sparse, and the existing literature does not address the clinical questions of patient safety posed by this review. We are therefore unable to draw any conclusions on safety or effectiveness. More primary research is needed to investigate optimal intubation techniques in obese patients, and new studies should be powered to detect differences in complications and in success rates rather than process measures such as speed, which are of limited clinical importance.

|
|
Camera Use Kink Resistance Reinforced Endotracheal Tube Vision Tube Images |